乳腺癌是女性最常见的恶性肿瘤之一[1],严重影响女性的身心健康,同时也给家庭及社会造成了比较大的危害。乳腺癌的发病因素很多,其中激素水平、遗传因素、居民生活习惯、食物内激素含量增加以及相关体内基因的变化与其密切相关。其主要牵涉到机体异常细胞的不可控的增长和转移,这其中涉及到很多与肿瘤相关基因的分子水平的变化[2-5]。因而从某程度上来说,寻找新的肿瘤治疗的分子靶点及开发分子靶向药物显得极为重要。星形细胞上调基因1(astrocyte elevated gene 1,AEG-1)在人类编码582个氨基酸,这些氨基酸序列在脊椎动物中是高度保守的,在调控不同的生理、病理过程中发挥显著的作用[2],特别是其在肿瘤的发生发展中发挥极其重要的作用。因此本研究通过观察AEG-1表达下调对乳腺癌细胞凋亡能力的影响,初步了解其在乳腺癌的发生发展过程中所发挥的作用及相关分子机制。
1 材料与方法
1.1 试剂与细胞株
AEG-1、caspase-3、caspase-9、β-actin兔抗人多克隆抗体、A E G-1 s i R N A、阴性对照siRNA、购自Santa Cruz公司。脂质体购自Invitrogen公司。MCF-7细胞购自中国科学院上海细胞研究所细胞库。其他常用剂由河南大学病理学实验室提供。
1.2 实验方法
1.2.1 细胞分组 空白对照组:未转染的乳腺癌MCF-7细胞;阴性对照组:用阴性对照siRNA转染的MCF-7细胞;AEG-1 siRNA组:利用AEG-1siRNA转染的MCF-7细胞。
1.2.2 细胞培养与基因转染 将液氮中冻存的MCF-7细胞加入RPMI-1640培养基,置于37 ℃,CO2培养箱中培养进行复苏后消化。中止消化后将培养瓶置于CO2培养箱中进行培养。待细胞达到指数生长期可以进行相应的实验。转染的前1 d,将一定数量的MCF-7细胞接种在RPMI-1640培养基中,当细胞的融合度达到85%~95%左右时,进行转染。按试剂盒说明制备siRNA和脂质体的混合物,并加入培养板中,在培养箱中孵育6 h后更换培养基,转染后48 h进行后续实验。
1.2.3 流式细胞术检测乳腺癌MCF-7细胞凋亡 收集转染48 h的3组乳腺癌MCF-7细胞至10 mL的离心管中,每组的细胞数大约为(1~5)×106/mL,然后于1 000 r/min的条件下离心、洗涤,于1 000 r/min离心5 min,于室温下避光孵育10~15 min,于1 000 r/min离心5 min以沉淀MCF-7细胞,并采用孵育缓冲液漂洗1次。加入荧光(SA-FLOUS)溶液于4 ℃条件下避光孵育20 min后,进行流式细胞仪分析检测。
1.2.4 Western blot 收集转染后48 h的3组乳腺癌MCF-7细胞。采用细胞裂解缓冲液提取总蛋白采用Bradford法测定蛋白浓度。计算上样量后电泳、转膜、封闭2 h。分别加一抗(AEG-1、caspase-3、caspase-9、β-actin)于含5%脱脂奶粉的洗涤缓冲液中,4 ℃过夜。第2天用洗涤缓冲液洗5 min×3次,分别加合适的二抗作用2 h,洗5 min×3次,然后曝光。将胶片进行拍照后,采用Gene Tools软件进行灰度值分析,计算蛋白的相对表达量,公式为:蛋白相对表达量=目的蛋白的表达量/内参蛋白的表达量。
1.3 统计学处理
所有结果采用SPSS 13.0软件进行统计学处理,两组数据间比较用独立样本t检验,计量资料采用均数±标准差(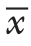 ±s)表示,组数据及以上行单因素方差分析(One-way ANOVA);P<0.05为差异有统计学意义。
±s)表示,组数据及以上行单因素方差分析(One-way ANOVA);P<0.05为差异有统计学意义。
2 结 果
2.1 AEG-1 siRNA干扰效果
Western blot检测3组乳腺癌MCF-7细胞中AEG-1蛋白的表达,结果表明,AEG-1 siRNA组中AEG-1蛋白的表达明显低于空白对照组和阴性对照组(均P<0.05),而空白对照组和阴性对照组之间相比,AEG-1蛋白的表达无差异(P>0.05)(图1)。提示AEG-1 siRNA能有效下调乳腺癌MCF-7中AEG-1蛋白的表达。

图1 Western blot检测AEG-1蛋白表达
Figure 1 Western blot analysis of AEG-1 protein expression
2.2 AEG-1表达下调对乳腺癌MCF-7细胞凋亡的影响
采用流式细胞术检测3组乳腺癌MCF-7细胞的细胞凋亡,结果显示,AEG-1 siRNA组中乳腺癌MCF-7细胞的早期、晚期凋亡率明显高于空白对照组和阴性对照组(均P<0.05),而空白对照组和阴性对照组之间乳腺癌MCF-7细胞的早期、晚期凋亡率均无统计学差异(均P>0.05)(图2)。为了进一步分析AEG-1 siRNA引发的乳腺癌MCF-7细胞的可能的分子机制,采用Western blot技术分析转染AEG-1 siRNA和对照siRNA前后细胞凋亡相关蛋白caspase-3和caspase-9的表达。结果显示,与空白对照组和阴性对照组相比,AEG-1 siRNA组中乳腺癌MCF-7细胞的caspase-3和caspase-9蛋白表达均明显上调(均P<0.05),而空白对照组和阴性对照组之间caspase-3和caspase-9蛋白表达无统计学差异(均P>0.05)(图3)。
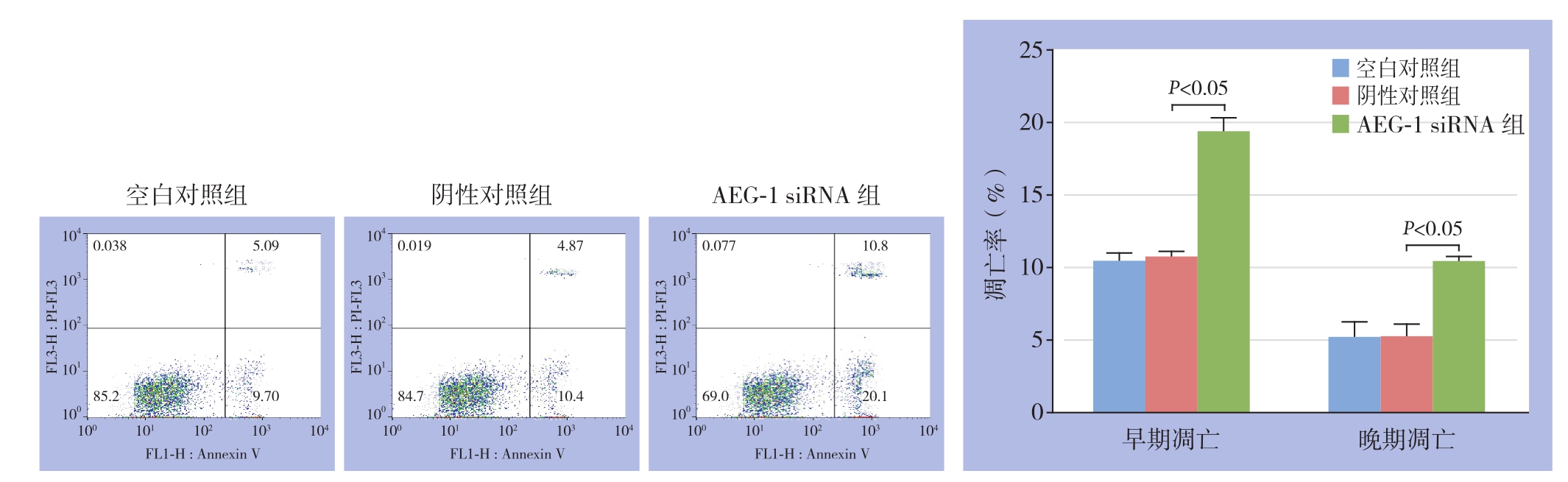
图2 流式细胞术检测胞凋亡情况
Figure 2 Flow cytometry analysis of cell apoptosis
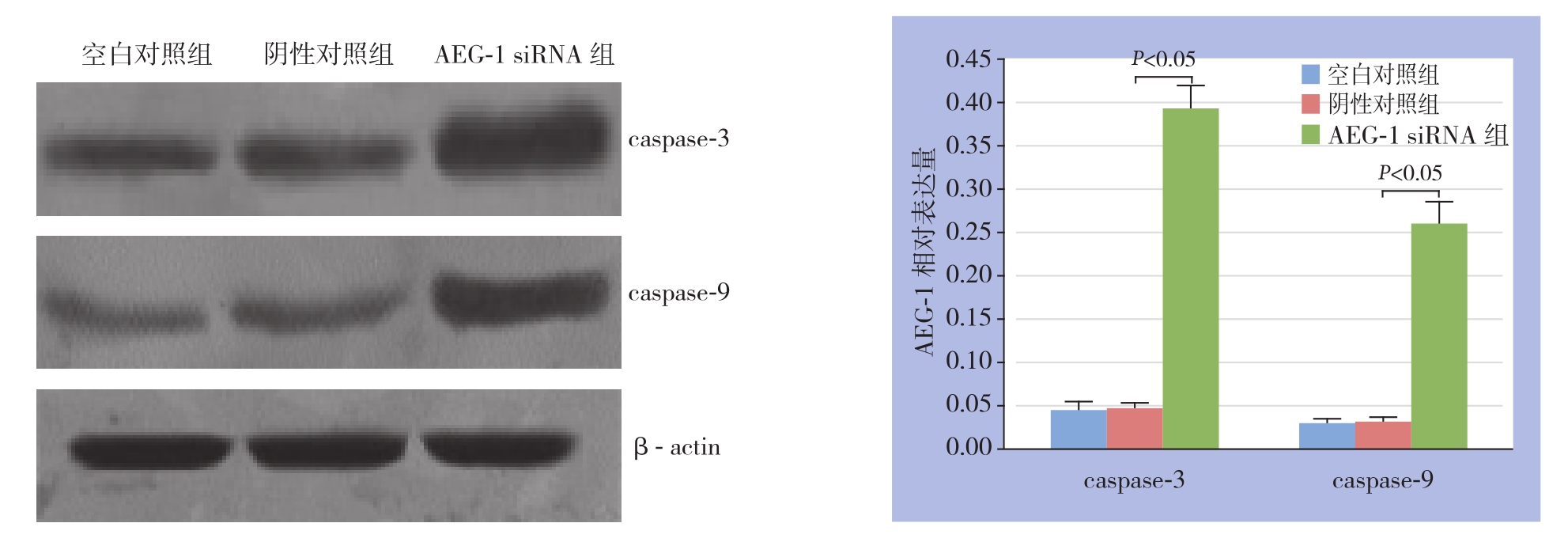
图3 Western blot检测各组细胞caspase-3和caspase-9蛋白的表达
Figure 3 Western blot analysis of expressions of caspase-3 and caspase-9
3 讨 论
积累的证据已经表明AEG-1在调控不同的生理、病理过程中发挥显著的作用,特别是其在肿瘤的发生发展中发挥极其重要的作用;与AEG-1在许多不同的恶性肿瘤中的作用一致,最近在许多肿瘤的临床研究中将AEG-1与肿瘤的进展和临床较差的预后密切联系在一起,提示AEG-1可能在肿瘤的发生、发展及进展中发挥及其重要的作用[6]。研究显示,AEG-1在多种不同的肿瘤中表达上调,包括:涎腺癌[7]、头颈部肿瘤[8-9]、胆囊癌[10]、卵巢癌[11-12]、非小细胞肺癌[13-14]、子宫内膜癌[15]、子宫颈癌[16-17]和肝癌[18]等。还有研究[19-22]证明,AEG-1在大多数正常的人类乳腺组织中低表达或缺失,但在许多乳腺癌细胞系或乳腺癌组织中呈现高表达。基于上述研究结果,究竟AEG-1在乳腺癌的发生发展中怎样发挥作用,其机制是什么,是否能应用于临床乳腺癌的治疗,目前国内研究尚少。当前兴起的RNA干扰技术是体内外研究基因功能较为理想的技术手段之一,许多研究[23-24]表明RNA干扰对于抑制肿瘤细胞中的癌基因的过度表达发挥重要作用,可能成为治疗肿瘤的一种新的手段和措施。因此上述研究表明,AEG-1在乳腺癌MCF-7细胞中高水平表达。在本研究中,将特异性的AEG-1 siRNA转染乳腺癌MCF-7细胞,采用Western blot技术研究乳腺癌MCF-7细胞中AEG-1蛋白的表达。结果发现,转染AEG-1siRNA后乳腺癌MCF-7细胞中AEG-1蛋白表达均显著低于空白对照组和阴性对照组(P<0.05),而空白对照组和阴性对照组之间相比,AEG-1蛋白表达无统计学差异(P>0.05),这一结果提示AEG-1 siRNA能明显下调乳腺癌MCF-7细胞中AEG-1蛋白的表达。有研究[25]显示沉默乳腺癌细胞MCF-7的AEG-1表达量,可以有效地抑制细胞增殖、迁移和侵袭的能力。该研究表明,AEG-1在乳腺癌的肿瘤的生长、局部扩散、组织浸润等过程中都发挥着重要作用;通过靶向沉默AEG-1,可以有效地抑制原发灶乳腺癌的生长和扩散。沉默乳腺癌细胞MCF-7的AEG-1表达量是否对乳腺癌细胞其他生物学行为同样存在影响,国内研究较少。
在其它的模型中,AEG-1也被证实抑制细胞凋亡,主要通过激活PI3K/AKT信号途径上调凋亡抑制基因的表达[26]。在正常星形细胞和纤维组织母细胞中AEG-1过表达诱导细胞凋亡[27]。当一组信号通路特异性的抑制剂被用来检测AEG-1前生存功能的下游调节子时,仅PI3K抑制剂LY294002、P T E N和显性负相A k t在无血清条件下能减弱AEG-1依赖的生存[28]。是否AEG-1表达下调能诱导乳腺癌MCF-7细胞发生凋亡呢?在本研究中,采用流式细胞术分析转染AEG-1 siRNA和对照siRNA前后乳腺癌MCF-7细胞凋亡的变化。结果发现,AEG-1 siRNA组中乳腺癌MCF-7细胞的凋亡率明显高于空白对照组和阴性对照组(P<0.05),而空白对照组和阴性对照组之间,乳腺癌MCF-7细胞的凋亡率无统计学差异(P>0.05),这一结果表明AEG-1表达下调能明显诱导乳腺癌MCF-7细胞发生凋亡。而凋亡效应分子caspase-3位于凋亡通路枢纽地位,caspase-3活化能引起细胞凋亡。caspase-3的活化主要依靠caspase-9的激活,caspase-3和caspase-9与乳腺癌发生发展密切相关[27]。为了进一步探讨其凋亡发生的可能的分子机制,本研究采用Western blot检测与细胞凋亡密切相关蛋白caspase-3和caspase-9的表达,结果发现AEG-1 siRNA组中caspase-3和caspase-9蛋白的表达显著高于空白对照组和阴性对照组(P<0.05),而空白对照组和阴性对照组之间caspase-3和caspase-9蛋白的表达无统计学差异(P>0.05),因而可以初步得出AEG-1表达下调引发的乳腺癌MCF-7细胞凋亡可能与caspase-3和caspase-9蛋白表达的上调密切相关,但是其详细的分子机制尚需要进一步探讨。
参考文献
[1]夏林玉.三阴性乳腺癌治疗的新进展[J].中国普通外科杂志,2016,25(5):741–746.doi:10.3978/j.issn.1005–6947.2016.05.020.Xia LY.Treatment of triple-negative breast cancer: resent progress[J].Chinese Journal of General Surgery,2016,25(5):741–746.doi:10.3978/j.issn.1005–6947.2016.05.020.
[2]肖顺崇,罗汉传,覃俊仕.miR-204对TFAM的靶向调控作用及其对乳腺癌细胞生长与增殖的影响[J].中国普通外科杂志,2016,25(11):1615–1621.doi:10.3978/j.issn.1005–6947.2016.11.016.Xiao SC,Luo HC,Qin JS.Targeted regulation of miR-204 on TFAM and their influence on growth and proliferation in breast cancer cells[J].Chinese Journal of General Surgery,2016,25(11):1615–1621.doi:10.3978/j.issn.1005–6947.2016.11.016.
[3]郭晨明,吴楠,郭丽英.我国HER-2阳性乳腺癌预后影响因素的Meta分析[J].中国普通外科杂志,2016,25(2):264–270.doi:10.3978/j.issn.1005–6947.2016.02.018.Guo CM,Wu N,Guo LY.Prognostic factors for HER-2-positive breast cancer in China: a Meta-analysis[J].Chinese Journal of General,2016,25(2):264–270.doi:10.3978/j.issn.1005–6947.2016.02.018.
[4]漆云翔,叶菲,宋晏琼.TGF-β RⅡ与HER-2阴性乳腺癌患者预后的关系研究[J].中国普通外科杂志,2016,25(5):756–760.doi:10.3978/j.issn.1005–6947.2016.05.023.Qi YX,Ye F,Song YQ.Study on relationship of TGF-β RⅡon prognosis of HER-2 negative breast cancer patients[J].Chinese Journal of General,2016,25(5):756–760.doi:10.3978/j.issn.1005–6947.2016.05.023.
[5]迟婷,姜晓燕.乳腺癌组织中miR-34a与VEGF的关系及临床意义[J].中国普通外科杂志,2016,25(5):680–685.doi:10.3978/j.issn.1005–6947.2016.05.010.Chi T,Jiang XY.Relationship between miR-34a and VEGF expressions in breast cancer tissue and the clinical significance[J].Chinese Journal of General,2016,25(5):680–685.doi:10.3978/j.issn.1005–6947.2016.05.010.
[6]Yoo BK,Emdad L,Lee SG,et al.Astrocyte elevated gene-1(AEG-1): A multifunctional regulator of normal and abnormal physiology[J].Pharmacol Ther,2011,130(1):1–8.doi: 10.1016/j.pharmthera.2011.01.008.
[7]Liao WT,Guo L,Zhong Y,et al.Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma[J].J Transl Med,2011,9:205.doi: 10.1186/1479–5876–9–205.
[8]Nohata N,Hanazawa T,Kikkawa N,et al.Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC)[J].J Hum Genet,2011,56(8):595–601.doi: 10.1038/jhg.2011.66.
[9]刘亮.AEG-1在头颈部肿瘤中的研究进展[J].医学综述,2014,20(5):813–816.doi:10.3969/j.issn.1006–2084.2014.05.015.Liu L.The Research Progress of AEG-1 in Head and Neck Cancer[J].Medical Recapitulate,2014,20(5):813–816.doi:10.3969/j.issn.1006–2084.2014.05.015.
[10]Sun W,Fan YZ,Xi H,et al.Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: an unfavorable and independent prognostic factor[J].Oncol Rep,2011,26(5):1133–1142.doi: 10.3892/or.2011.1387.
[11]Li C,Liu J,Lu R,et al.AEG -1 overexpression: a novel indicator for peritoneal dissemination and lymph node metastasis in epithelial ovarian cancers[J].Int J Gynecol Cancer,2011,21(4):602–608.doi:10.1097/IGC.0b013e3182145561.
[12]张淼,周秀春,张冠群,等.AEG-1在卵巢癌中的表达及其与临床病理因素的关系[J].现代医药卫生,2015,31(13):2004–2005.doi:10.3969/j.issn.1009–5519.2015.13.029.Zhang M,Zhou XC,Zhang GQ,et al.AEG-1 expression in ovarian cancer and its relation with clincopathologic factors[J].Journal of Modern Medicine & Health,2015,31(13):2004–2005.doi:10.3969/j.issn.1009–5519.2015.13.029.
[13]马志红,陈莹蓉,董顺利,等.NSCLC中AEG-1的表达及与临床病理特征和血管生成的关系研究[J].浙江医学,2017,39(8):617–619.doi:10.12056/j.issn.1006–2785.2017.39.8.2017–293.Ma ZH,Chen YR,Dong SL,et al.Expression of AEG-1 in nonsmall cell lung cancer and its relation to clinicopathological features and angiogenesis[J].Zhejiang Medical Journal,2017,39(8):617–619.doi:10.12056/j.issn.1006–2785.2017.39.8.2017–293.
[14]高建华,曹华,李萱,等.AEG-1基因在NSCLC中的表达及其临床病理意义[J].中国免疫学杂志,2016,32(12):1833–1836.doi:10.3969/j.issn.1000–484X.2016.12.024.Gao JH,Cao H,Li X,et al.Expression of AEG-1–1 gene in NSCLC and its clinical significance[J].Chinese Journal of Immunology,2016,32(12):1833–1836.doi:10.3969/j.issn.1000–484X.2016.12.024.
[15]Song H,Li C,Lu R,et al.Expression of astrocyte elevated gene-1:a novel marker of the pathogenesis,progression,and poor prognosis for endometrial cancer[J].Int J Gynecol Cancer,2010,20(7):1188–1196.
[16]宋恩霖,王茂梅,熊秀娟,等.子宫颈癌组织中AEG-1表达与微血管生成的关系[J].临床与实验病理学杂志,2014,30(7):717–720.doi:10.13315/j.cnki.cjcep.2014.07.003.Song EL,Wang MM,Xiong XJ,et al.Expression of AEG-1 and relationship with angiogenesis in cervical carcinoma[J].Chinese Journal of Clinical and Experimental Pathology,2014,30(7):717–720.doi:10.13315/j.cnki.cjcep.2014.07.003.
[17]赵娟,马唯,吴林林,等.AEG-1、P16、KI-67蛋白在宫颈癌前病变中的表达与临床意义[J].中国性科学,2016,25(10):35–39.dio:10.3969/j.issn.1672–1993.2016.10.011.Zhao J,Ma W,Wu LL,et al.The expression of AEG- 1,P16,KI-67 protein in precancerous cervical lesions and its clinical significance[J].The Chinese Journal of Human Sexuality,2016,25(10):35–39.dio:10.3969/j.issn.1672–1993.2016.10.011.
[18]程云娟,罗新华,程明亮.AEG-1、β-catenin和C-myc在原发性肝细胞癌中的临床特性与分析[J].标记免疫分析与临床,2015,22(7):633–636.doi:10.11748/bjmy.issn.1006–1703.2015.07.013.Cheng YJ,Luo XH,Cheng ML.Clinical Feature Analysis of AEG-1,β-catenin and C-myc Expression in Primary Hepatocellular Carcinoma[J].Labeled Immunoassays and Clinical Medicine,2015,22(7):633–636.doi:10.11748/bjmy.issn.1006–1703.2015.07.013.
[19]Brown DM,Ruoslahti E.Metadherin,a cell surface protein in breast tumors that mediates lung metastasis[J].Cancer Cell,2004,5(4):365–374.
[20]Hu G,Chong RA,Yang Q,et al.MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poorprognosis breast cancer[J].Cancer Cell,2009,15(1):9–20.doi:10.1016/j.ccr.2008.11.013.
[21]Li J,Zhang N,Song LB,et al.Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival[J].Clin Cancer Res,2008,14(11):3319–3326.doi:10.1158/1078–0432.CCR-07–4054.
[22]曲美华,董秀玲,朴正爱,等.乳腺癌AEG-1、C-erbB2和VEGF的表达及临床研究[J].中国保健营养,2015,25(17):46–47.Qu MH,Dong XL,Piao ZA,et al.Expressions of AEG-1,C-erbB2 and VEGF in breast cancer and associated clinical study[J].China Health Care & Nutrition,2015,25(17):46–47.
[23]刘言,李双齐,刘景波.星形细胞上调基因-1及Girdin在乳腺癌组织中的表达[J].中华实验外科杂志,2015,32(11):2818–2819.doi:10.3760/cma.j.issn.1001–9030.2015.11.058.Liu Y,Li SQ,Liu JB.Expression of astrocyte elevated gene-1 and Girdin in breast carcinoma[J].Chinese Journal of Experimental Surgery,2015,32(11):2818–2819.doi:10.3760/cma.j.issn.1001–9030.2015.11.058.
[24]Xu C,Lee SA,Chen X.RNA interference as therapeutics for hepatocellular carcinoma[J].Recent Pat Anticancer Drug Discov,2011,6(1):105–115.
[25]李英红,刘珺珺,宁晓明,等.AEG-1基因促进乳腺癌细胞株MCF-7转移[J].实用肿瘤学杂志,2017,31(4):299–304.dio:10.11904/j.issn.1002–3070.2017.04.003.Li YH,Liu JJ,Ning XM,et al.AEG-1 promotes metastasis of breast cancer MCF-7 cells[J].Practical Oncology Journal,2017,31(4):299–304.dio: 10.11904/j.issn.1002–3070.2017.04.003.
[26]Spirin PV,Baskaran F,Orlova NN,et al.Downregulation of activated leukemic oncogenes AML1-ETO and RUNX1(K83N)expression with RNA-interference[J].Mol Biol (Mosk),2010,44(5):876–888.
[27]王进京,孙保存.乳腺癌中caspase-3和caspase-9的表达及其意义[J].临床与实验病理学杂志,2012,28(4):378–381.doi:10.3969/j.issn.1001–7399.2012.04.006.Wang JJ,Sun BC.Expression of caspase-3 and caspase-9 and their significance in breast cancer[J].Chinese Journal of Clinical and Experimental Pathology,2012,28(4):378–381.doi:10.3969/j.issn.1001–7399.2012.04.006.
[28]Lee SG,Su ZZ,Emdad L,et al.Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling[J].Oncogene,2008,27(8):1114–1121.dio: 10.1038/sj.onc.1210713.
