胆管癌(CCA)是来自胆管上皮的恶性肿瘤。胆管癌的发生率在过去10年都有增高的趋势,但是在个别地区胆管癌的发生率在过去10年却翻了一番。其预后差,5年无瘤生存率仅为12.8%,中位生存时间仅1.5年[1-2]。胆管癌占消化系统恶性肿瘤的3%,是第二最常见的主要肝胆肿瘤[3]。胆管癌患者初始症状取决于肿瘤位置。肝内胆管癌通常表现为非特异性疾病的晚期阶段[4]。通常,胆管癌患者中仅在少数患者(15%~30%)中实现细胞学诊断[5-6]。一般来说,胆管癌患者病死率高,因为在诊断时已经到了晚期[7]。不同系统癌症预后影响因素包括TNM分期、手术切缘状态等被用来作为胆管癌患者手术后的预后指标[8-12]。但胆管癌的发生与发展的机制尚未完全探明[13-15]。寻找胆管癌新的、有效的早期诊断生物学指标和预测胆管癌发生转移、复发以及预后的分子标志物显得十分必要。
靶向Xklp2靶蛋白(targeting protein for xenopus kinesin-like protein 2,TPX2)是一种严格受细胞周期调控的微管相关蛋白,在有丝分裂纺锤体形成过程中发挥重要作用[16]。研究发现TPX2在肝癌、胃癌、胰腺癌、肺癌、宫颈癌、膀胱癌、食管鳞状细胞癌中都存在过表达,导致细胞基因的的损伤、中心体异常扩增及细胞恶性转化,进而促进细胞增殖,抑制细胞凋亡,阻滞细胞周期于G2/M期,并与肿瘤分化、转移和复发相关[17-23]。但有关TPX2在胆管癌癌组织中的表达水平及胆管癌中的表达水平与临床预后的关系未见相关报道。因此,本研究应用免疫组化技术和Western blot技术检测胆管癌患者癌组织和癌旁组织中的TPX2蛋白的表达情况,收集因胆管癌手术的患者的临床病理资料,探寻其在胆管癌的发生与发展过程中的可能作用。
1 材料与方法
1.1 标本来源
收集2010年1月—2012年1月期间在南昌大学第二附属医院肝胆外科行胆管癌切除术且术后均经病理科2位病理科医师证实为胆管癌的60例患者的癌组织和非癌组织(胆管或肝脏)标本。在距肿瘤边缘1 cm以内且无坏死的肿瘤组织取胆管癌组织,在距肿瘤边缘至少3 cm的组织取非癌组织,经病理证实无肿瘤细胞。所有患者术前均未经过放、化疗。癌细胞分化为高、中、低,胆管癌的临床分期根据美国癌症联合委员会(AJCC)的TNM分期为I~IV期。
1.2 主要试剂及仪器
小鼠抗人TPX2单克隆抗体购自美国BioLegend公司(B159993),一抗稀释液购自武汉市博士的生物工程有限公司,通用型二步检测法试剂盒(PV-6000)、二氡基联苯胺(DAB)显色剂均购自北京中杉生物技术有限公司。
1.3 免疫组化检测
胆管癌癌组织和癌旁组织经10%中性甲醛固定24 h,石蜡包埋后,切成约3 μm切片。石蜡切片经60 ℃烤片1.5 h,脱蜡,水化,高压抗原修复10 min,3%过氧化氢阻断内源性过氧化物酶30 min,孵育一抗(1:200)4 ℃过夜,次日二抗孵育1 h,DAB)显色,苏木精复染后封片。
1.4 免疫组化结果判定
由2位病理科医师独立阅片;TPX2蛋白的表达部位为细胞核,阳性细胞核被染色为浅棕色至深褐色,其染色强度评判标准以阳性细胞核占全部细胞核的百分率界定,具体为:无核染色为(-);核染色≤10%为(+);11%~30%为(++);31%~50%为(+++);>50%为(++++);(++)以上被判定为阳性表达。
1.5 Western blot检测
取收集的标本出来清洗好,放入匀浆器中于冰上加入含蛋白酶体抑制剂的RIPA蛋白裂解液,裂解,于4 ℃ 10 000 r/min离心15 min,取上清液。加入抽提试剂,在4 ℃冰箱静置,加入2×上样缓冲液,95 ℃ 5 min煮沸变性。10%聚丙烯酰胺凝胶电泳,转膜至PVDF膜,5%脱脂奶粉封闭2 h。一抗4 ℃孵育过夜,使用对应属性的二抗孵育1 h,ECL发光试剂盒显影,暗室中X光片曝光成像。
1.6 统计学处理
用SPSS 17.0软件对实验数据进行处理,病理相关性分析采用Pearson检验,以α=0.05为检验水准。

图1 免疫组化检测TPX2蛋白(×400) A:胆管癌组织TPX2蛋白阳性表达;B:癌旁组织TPX2蛋白阴性表达
Figure 1 Immunohistochemical staining for TPX2 protein (×400) A: Positive expression of TPX2 protein in cholangiocarcinoma tissue;B: Negative expression of TPX2 protein in adjacent tissue
2 结 果
2.1 胆管癌癌组织与癌旁组织TPX2蛋白表达水平的检测
运用免疫组化检测60例胆管癌癌组织与癌旁组织TPX2蛋白表达水平,结果显示,在胆管癌癌旁组织中TPX2蛋白阳性表达率为8.3%(5/60),在胆管癌癌组织中TPX2蛋白阳性表达率为46.7%(28/60),差异有统计学意义(P<0.05)(图1)。同时随机选取4对胆管癌患者的标本的癌组织和癌旁组织提取组织蛋白,通过Western blot检测胆管癌癌组织与癌旁组织TPX2蛋白表达水平,结果显示TPX2蛋白在癌组织中表达水平高于癌旁组织(图2)。
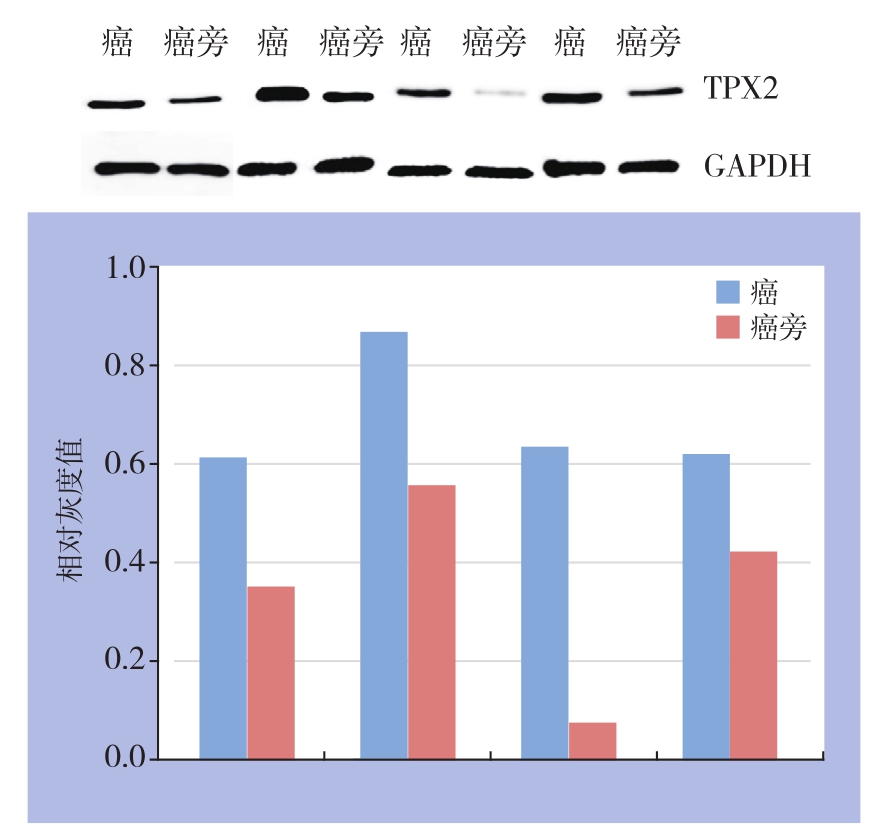
图2 Western blot检测TPX2的蛋白表达量
Figure 2 Western blot analysis for TPX2 protein expression level
2.2 TPX2蛋白的表达水平与临床病理参数之间的关系
分析免疫组化TPX2蛋白的表达水平与对应患者临床数据的病理参数之间的关系。结果显示:TPX2蛋白的表达水平与TNM分期、淋巴结转移以及患者生存有关(均P<0.05)。但是与患者的性别、年龄、肿瘤大小、病理分化程度、CA19-9表达水平等无关(均P>0.05)(表1)。
2.3 TPX2蛋白的表达水平与胆管癌患者术后生存期的关系
对术后60例患者获随访,随访时间12~48个月。Kaplan-Meier生存曲线显示术后生存时间TPX2蛋白阳性组比TPX2蛋白阴性组生存时间明显缩短。两组患者总生存率的比较,差异均有统计学意义(2年生存率:27.6% vs. 78.4%,3年生存率:14.3% vs. 59.5%,均P<0.05)(图3)。
表1 TPX2蛋白表达与胆管癌患者临床病理参数之间的关系[n(%)]
Table 1 Relations of TPX2 protein expression with clinical factors of cholangiocarcinoma patients [n (%)]

因素 n TPX2阳性 TPX2阴性 χ2 P性别男 34 18(52.9) 16(47.1) 1.241 0.305女 26 10(38.5) 16(61.5)年龄(岁)>55 32 16(50.0) 16(50.0) 0.306 0.613≤55 28 12(37.1) 16(57.1)肿瘤大小(cm)≤2.5 20 8(40.0) 12(60.0) 0.536 0.585>2.5 40 20(50.0) 24(50.0)CA19-9水平(U/mL)≤37 27 12(44.4) 15(55.6) 0.097 0.799>37 33 16(48.5) 17(51.5)病理分化程度低分化 26 14(53.8) 12(46.2) 0.695 0.470中分化 34 14(41.2) 20(58.8)生存状况存活 29 8(27.6) 21(72.4) 8.210 0.005死亡 31 20(64.5) 11(35.5)TNM分期I~II 31 10(32.3) 21(67.7) 5.350 0.037 III~IV 29 18(62.1) 11(37.9)N分期N0 25 8(32.0) 17(68.0) 6.605 0.009 N1~N3 35 20(57.1) 15(42.9)
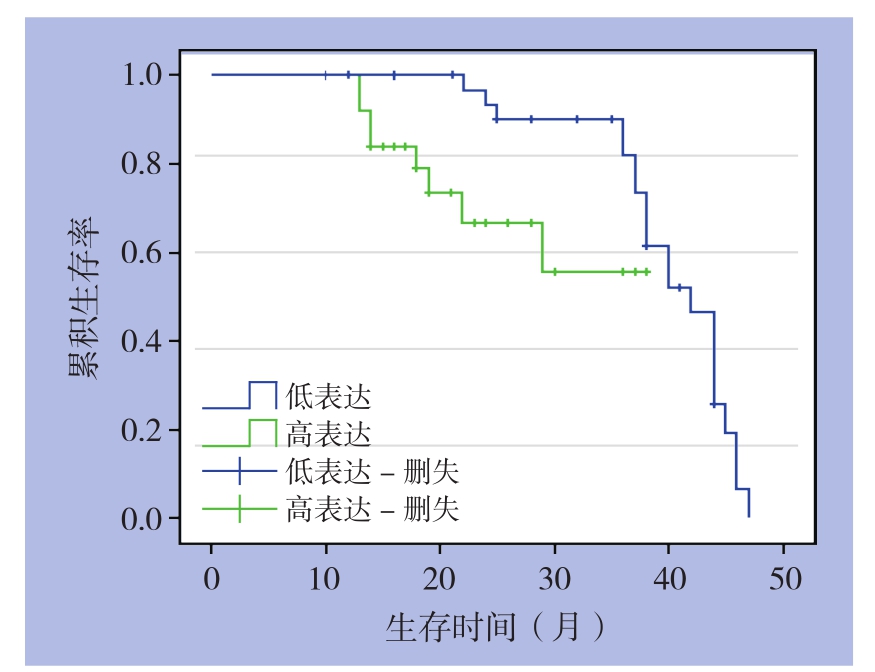
图3 TPX2阳性患者(28例)和阴性患者(32例)生存曲线比较
Figure 3 Comparison of the survival curves between patients with positive TPX2 expression (28 cases) and those with negative TPX2 expression (32 cases)
3 讨 论
早期诊断和完全手术切除为胆管癌患者提供了治愈的机会,但目前缺乏早期筛查和诊断胆管癌有效方法,对于胆管癌晚期患者尚无有效的治疗方法[24]。胆管癌被认为是一种对放、化疗不敏感的肿瘤。胆管癌比其他胃肠道肿瘤对化疗敏感性差[25-26]。分子蛋白的鉴定和基因序列的测定可以帮助临床医生早期筛查和诊断胆管癌的患者,也为晚期癌症患者提供靶向治疗。TPX2是微管相关蛋白。TPX2过表达促进微管形成。TPX2可从细胞核逃逸以调节非有丝分裂细胞的微管细胞骨架[27]。TPX2可以作为影响细胞周期的独立因素。TPX2的过表达与胰腺癌的分级,浸润,淋巴转移和远处转移有关[19]。目前认为TPX2可能通过影响细胞增殖、周期、凋亡及迁移和侵袭能力来影响肿瘤的发生发展[17-23]。当用TPX2siRNA干扰细胞TPX2蛋白表达时,促进细胞的凋亡;将细胞周期阻滞于G2/M期而不能进行正常的有丝分裂;抑制细胞的迁移和侵袭能力[17-25]。然而TPX2表达在胆管癌中罕有研究。
本研究中,在胆管癌癌旁组织中TPX2蛋白阴性表达率为91.7%(55/60),在胆管癌癌组织中TPX2蛋白阳性表达率为46.7%(28/60);通过Western blot技术检测胆管癌癌组织与癌旁组织TPX2蛋白表达情况,结果示:TPX2蛋白在胆管癌组织中的表达水平显著高于癌旁组织。说明TPX2蛋白在胆管癌组织中高表达。运用统计学方法分析TPX2蛋白与患者临床病理参数之间的关系,结果示:TPX2蛋白的表达水平与淋巴结转移 、TNM分期以及患者生存期有关;与年龄、性别、病理分化程度、CA199和肿瘤大小无关。术后无瘤生存时间TPX2蛋白阳性组比TPX2蛋白阴性组无瘤生存时间明显缩短。因此本研究可以得出结论:胆管癌患者癌组织中TPX2高表达提示预后不良。
综上所述,本研究显示TPX2胆管癌组织中处于高表达,是胆管癌患者术后发生转移 、复发的危险因素。TPX2可能是评价胆管癌患者预后的一个潜在生物学指标,TPX2有可能作为分子标记成为胆管癌免疫治疗的潜在靶点。其影响胆管癌的发生和发展的具体机制尚不清楚,还需要多实验研究进行验证。
参考文献
[1] 周少君, 黄志勇. 肝内胆管癌根治性切除术后肿瘤复发转移的预后因素分析[J]. 中国普通外科杂志, 2014, 23(8):1024–1029.doi:10.7659/j.issn.1005–6947.2014.08.002.Zhou SJ, Huang ZY. Prognostic factors for tumor recurrence and metastasis of intrahepatic cholangiocarcinoma after radical resection[J]. Chinese Journal of General Surgery, 2014, 23(8):1024–1029. doi:10.7659/j.issn.1005–6947.2014.08.002.
[2] Razumilava N, Gores GJ. Cholangiocarcinoma[J]. Lancet, 2014,383(9935):2168–2179. doi: 10.1016/S0140–6736(13)61903–0.
[3] Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma[J].Lancet, 2005, 366(9493):1303–1314.
[4] Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma[J]. J Hepatol, 2014, 60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021.
[5] Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures[J]. Am J Gastroenterol, 2004,99(9):1675–1681.
[6] Selvaggi SM. Biliary brushing cytology[J]. Cytopathology, 2004,15(2):74–79.
[7] Ahrendt SA, Nakeeb A, Pitt HA. Cholangiocarcinoma[J]. Clin Liver Dis, 2001, 5(1):191–218.
[8] Saxena A, Chua TC, Chu FC, et al. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival[J]. Am J Surg, 2011,202(3):310–320. doi: 10.1016/j.amjsurg.2010.08.041.
[9] Saxena A, Chua TC, Sarkar A, et al. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit[J]. J Gastrointest Surg,2010, 14(7):1128–1138. doi: 10.1007/s11605–010–1203–1.
[10] Hidalgo E, Asthana S, Nishio H, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience[J]. Eur J Surg Oncol,2008, 34(7):787–794.
[11] Jonas S, Thelen A, Benckert C, et al. Extended liver resection for intrahepatic cholangiocarcinoma: A comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification[J]. Ann Surg, 2009, 249(2):303–309. doi: 10.1097/SLA.0b013e318195e164.
[12] de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment[J]. J Clin Oncol,2011, 29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519.
[13] Gera S, Ettel M, Acosta-Gonzalez G, et al. Clinical features,histology, and histogenesis of combined hepatocellularcholangiocarcinoma[J]. World J Hepatol, 2017, 9(6):300–309.doi:10.4254/wjh.v9.i6.300.
[14] Vogel A, Saborowski A. Cholangiocellular Carcinoma[J]. Digestion,2017, 95(3):181–185. doi: 10.1159/000454763.
[15] Maroni L, Pierantonelli I, Banales JM, 等. 遗传学在胆管细胞癌发展中的重要性[J].中国普通外科杂志, 2015, 24(2):151–162.doi:10.3978/j.issn.1005–6947.2015.02.001.Maroni L, Pierantonelli I, Banales JM, et al. The significance of genetics for cholangiocarcinoma development[J]. Chinese Journal of General Surgery, 2015, 24(2):151–162. doi:10.3978/j.issn.1005–6947.2015.02.001.
[16] Neumayer G, Belzil C, Gruss OJ, et al. TPX2: of spindle assembly,DNA damage response, and cancer[J]. Cell Mol Life Sci, 2014,71(16):3027–3047. doi: 10.1007/s00018–014–1582–7.
[17] Liang B, Jia C, Huang Y, et al. TPX2 Level Correlates with Hepatocellular Carcinoma Cell Proliferation, Apoptosis, and EMT[J]. Dig Dis Sci, 2015, 60(8):2360–2372. doi: 10.1007/s10620–015–3730–9.
[18] Liang B, Zheng W, Fang L, et al. Overexpressed targeting protein for xklp2 (tpx2) serves as a promising prognostic marker and therapeutic target for gastric cancer[J]. Cancer Biol Ther, 2016,17(8):824–832. doi: 10.1080/15384047.2016.1195046.
[19] Warner SL, Stephens BJ, Nwokenkwo S, et al. Validation of TPX2 as a potential therapeutic target in pancreatic cancer cells [J]. Clin Cancer Res, 2009, 15(21):6519–6528. doi: 10.1158/1078–0432.CCR–09–0077.
[20] Ma Y, Lin D, Sun W, et al. Expression of targeting protein for xklp2 associated with both malignant transformation of respiratory epithelium and progression of squamous cell lung cancer[J]. Clin Cancer Res, 2006, 12(4):1121–1127.
[21] Chang H, Wang J, Tian Y, et al. The TPX2 gene is a promising diagnostic and therapeutic target for cervical cancer[J]. Oncol Rep,2012, 27(5):1353–1359. doi: 10.3892/or.2012.1668.
[22] Yan L, Li S, Xu C, et al. Target protein for Xklp2 (TPX2), a microtubule-related protein, contributes to malignant phenotype in bladder carcinoma [J]. Tumour Biol, 2013, 34(6):4089–4100. doi:10.1007/s13277–013–1000-z.
[23] Hsu PK, Chen HY, Yeh YC, et al. TPX2 expression is associated with cell proliferation and patient outcome in esophageal squamous cell carcinoma[J]. J Gastroenterol, 2014, 49(8):1231–1240. doi:10.1007/s00535–013–0870–6.
[24] Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis:absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma[J].Hepatology, 2009, 50(3):791–798. doi: 10.1002/hep.23071.
[25] Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type[J]. J Hepatobiliary Pancreat Sci,2015, 22(2):94–100. doi: 10.1002/jhbp.154.
[26] Tabrizian P, Jibara G, Hechtman JF, et al. Outcomes following resection of intrahepatic cholangiocarcinoma[J]. HPB (Oxford),2015, 17(4):344–351. doi: 10.1111/hpb.12359.
[27] Wadsworth P. Tpx2[J]. Curr Biol, 2015, 25(24):R1156–1158. doi:10.1016/j.cub.2015.10.003.
