胃癌是世界常见的恶性肿瘤之一,在2015年中国的癌症发病率与致死率统计中高居第2位[1]。在亚洲,进展期胃癌的治疗方案主要是根治性手术联合辅助化疗。多样的联合化疗方案已被证实具备有效性[2-3]。在日本,替吉奥联合顺铂新辅助化疗方案已经被广泛应用于III期胃癌患者[4]。尽管胃癌患者的总体生存率在联合治疗下有所提高,但化疗未缓解者常常无法及时接受更为敏感的化疗药物治疗[5]。因此,通过差异基因表达谱来判断新辅助化疗药物的敏感性显得尤为重要。本研究主要关注替吉奥联合奥沙利铂(SOX)新辅助化疗不同疗效所引起的差异基因表达情况。以术后大体标本作为研究对象,分为缓解组、未缓解组及未行新辅助化疗者3组进行两两比较。对于SOX方案而言,替吉奥与奥沙利铂的耐药性主要与叶酸代谢及DNA损伤修复有密切关系。本研究以DNA损伤修复和叶酸代谢为重点,分析基因表达谱与胃癌化疗药物的耐药性的关系。
1 资料与方法
1.1 一般资料
按照TNM分期[6],所有患者均被临床诊断为局部进展期胃癌(T3/T4,N+,M0)。纳入及排除标准基于笔者之前的研究结果[7]。患者需接受4个周期的新辅助化疗方案。D2根治术是以日本胃癌研究学会指南作为标准[8]。依据RECIST 1.1标准[9]及大体组织标本判断肿瘤疗效,6例缓解者组织样本作为缓解组;6例未缓解者组织样本为未缓解组;3例未行新辅助化疗者组织样本为未化疗组。
1.2 高通量基因芯片技术
按TRIzol方法抽提胃癌组织R N A,采用RNeasy Mini Kit进一步纯化RNA,应用琼脂糖凝胶电泳判断28S和18S的核糖体峰值比来评价RNA的质量。利用Affymetrix公司的GeneChip PrimeView人类基因表达列阵对15例手术组织标本进行分析。采用表达谱芯片配套试剂盒GeneChip 3'IVT Express Kit说明和标准操作流程对样品RNA进行进一步放大、标记和纯化。采用基因芯片扫描仪3000进行信号收集。通过信号直方图、相对信号箱图、皮尔森相关性及主成分分析来保障基因芯片的质量。
1.3 数据分析
采用Gene Math 2.0软件进行聚类分析,对缓解组、未缓解组及未化疗组的上调或下调基因进行比较。应用Gene Ontology(GO)分析对表达基因进行分类[10]。根据美国生物技术信息国立中心的重要功能分类,可以把GO分为分子功能、生物学途径及细胞组件3部分。基于SOX方案的耐药机制,DNA损伤修复(GO:0006281)和叶酸代谢(GO:0006766)作为重点的分析对象,筛选出相关的差异基因。在确定差异基因基础上,通过KEGG来解释每个差异表达基因所在通路[11]。以P<0.05判断通路显著性,筛选有意义的目标通路。
1.4 统计学处理
采用SPSS 19.0软件及Excel 2010进行数据分析。应用ANONA和χ2检验对缓解组、未缓解组及未化疗组之间进行多重比较。Fisher检验和χ2检验主要针对GO分类进行研究。差异倍数(FC)为待比较两个指标标准化后信号的比值,本研究差异基因筛选的阈值设定为FC>1.5。
2 结 果
2.1 基因表达分析
6例缓解者、6例未缓解者与3例未行化疗样本3组进行差异基因识别。为了证实3组直接的关系,对基因表达数据进行聚类分析。在缓解组与未缓解组中,共识别458个上调基因和241个下调基因。缓解组与未化疗组进行比较,发现108个上调基因和283个下调基因。而未缓解组与未化疗组比较时,共有172个上调基因和85个下调基因。为了能够更好地理解SOX新辅助化疗耐药性的分子机制,利用GO分类和KEGG进行功能和通路分析。
2.2 功能分析
在新辅助化疗中,主要GO分子功能与生物学途径进行基因表达分析。缓解组与未缓解组中,GO生物学途径的分析结果主要集中在信号转导、多细胞有机体发育、生物代谢过程、RNA代谢过程及蛋白代谢过程。GO分子功能主要是受体活性、跨膜受体活性、低密度脂蛋白结合、钙离子结合及跨膜转运蛋白活性(表1)。由此可知,SOX方案的耐药性与受体相互作用、信号通路、细胞代谢、跨膜转运等有所关联。
缓解组与未化疗组进行比较时,GO生物学途径主要是信号转导、多细胞有机体发育及细胞大分子代谢过程。GO分子功能是酶调节活性、激酶活性、蛋白激酶活性及受体结合。该结果表明,新辅助化疗肿瘤组织的敏感性与化疗所致药理作用可能会使两者间存在差异。对未缓解组与未化疗组进行比较,但GO生物学途径和分子功能均没有得到有意义的数据。
表1 缓解组与未缓解组GO分子功能与生物学途径的基因表达分析
Table 1 Analysis of gene expressions related to the GO ontologies of molecular function and biological process of the responder group and non-responder group

G O分类 G O-I D P 表达发生变化的基因总数 G O分析差异基因数 描述生物学途径 0 0 0 7 1 6 5 0.0 0 0 0 0 0 0 0 0 0 0 3 2 9 1 6 3 4 6 9 信号转导0 0 0 7 2 7 5 0.0 0 0 0 0 0 0 0 0 0 1 1 1 1 0 4 9 5 2 多细胞有机体发育0 0 4 3 2 8 3 0.0 0 0 0 0 0 0 0 0 1 3 7 1 6 8 4 6 6 生物代谢过程0 0 0 6 1 3 9 0.0 0 0 0 0 0 0 0 2 8 5 1 2 4 4 5 2 核酸代谢过程0 0 4 8 8 5 6 0.0 0 0 0 0 0 0 0 2 8 5 1 0 1 3 4 6 解剖结构发展0 0 4 8 5 1 3 0.0 0 0 0 0 0 0 0 4 2 6 5 7 1 3 3 器官发展0 0 4 8 7 3 1 0.0 0 0 0 0 0 0 2 0 6 8 6 1 4 0 系统发展0 0 4 4 2 6 0 0.0 0 0 0 0 0 0 2 0 6 1 1 3 1 4 7 细胞大分子代谢过程0 0 1 6 0 7 0 0.0 0 0 0 0 0 0 2 8 5 8 4 1 3 9 R N A代谢过程0 0 1 9 5 3 8 0.0 0 0 0 0 0 0 2 8 5 1 2 3 1 4 9 蛋白代谢过程分子功能 0 0 0 4 8 7 2 0.0 0 0 0 0 5 6 5 8 3 2 9 受体活性0 0 0 4 8 8 8 0.0 0 0 0 1 4 7 4 1 8 2 3 跨膜受体活性0 0 2 2 8 9 2 0.0 0 0 6 3 9 2 1 9 底物特异性转运蛋白活性0 0 1 6 4 6 2 0.0 0 0 6 2 2 6 1 4 焦磷酸酶活性0 0 1 6 8 1 7 0.0 0 0 6 2 2 8 1 4 水解酶活性0 0 2 2 8 9 1 0.0 0 0 9 8 3 3 4 4 1 7 跨膜转运活性0 0 3 0 1 6 9 0.0 0 1 1 1 1 2 4 低密度脂蛋白结合0 0 0 5 5 0 9 0.0 0 1 1 1 1 0 4 9 钙离子结合0 0 5 1 0 8 2 0.0 0 1 3 8 4 2 6 未折叠蛋白结合0 0 2 2 8 5 7 0.0 0 1 7 3 7 5 1 7 跨膜转运活性
2.3 通路分析
以KEGG公共Pathway数据库的信息,分析SOX新辅助药物耐药性的差异基因所在通路及某个Pathway中基因富集度的显著性水平。在缓解组与未缓解组中,差异基因主要与NK细胞介导细胞毒性、细胞因子配体受体相互作用有关。其具有统计学差异的目标通路主要涉及受体相互作用与信号通路等方面。而缓解组与未化疗组比较时,表达基因主要与MAPK信号通路、细胞凋亡及Toll样受体信号通路有关。
2.4 SOX方案耐药相关生物标记物
在缓解组和未缓解组中,HUS1、RECQL5及XRCC4可能与该类药物耐药性有关(表2)。缓解组与未缓解组比较,GADD45G下调显著;而在缓解组与未化疗组中,该基因上调显著。BTG2仅在缓解组与未化疗组对比中出现下调。在未缓解组与未化疗组比较中,DDB2明显下调。对于叶酸代谢而言,在3组比较中,本研究未找到任何差异基因。
表2 DNA损伤修复相关的差异基因
Table 2 Differentially expressed genes associated with DNA damage repair
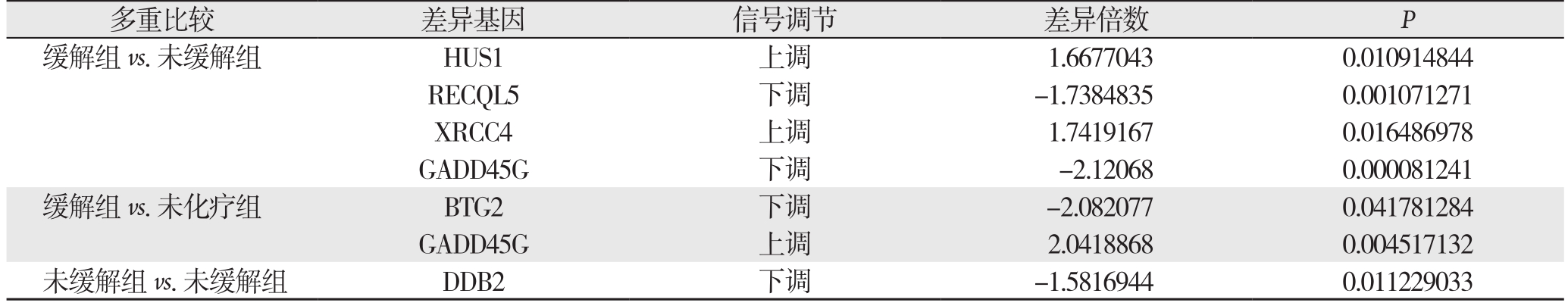
多重比较 差异基因 信号调节 差异倍数 P缓解组vs.未缓解组 HUS1 上调 1.6677043 0.010914844 RECQL5 下调 -1.7384835 0.001071271 XRCC4 上调 1.7419167 0.016486978 GADD45G 下调 -2.12068 0.000081241缓解组vs.未化疗组 BTG2 下调 -2.082077 0.041781284 GADD45G 上调 2.0418868 0.004517132未缓解组vs.未缓解组 DDB2 下调 -1.5816944 0.011229033
3 讨 论
最近几十年中,新辅助化疗成为进展期胃癌的标准治疗手段之一。多项临床研究[12-13]表明术前化疗比术后治疗具有一定优势。在这些研究中,由于DNA损伤修复及叶酸代谢机制,某些患者对化疗药物产生耐药性,而无法从治疗方案中获益。因此,为了能够提供最佳的治疗方案,采用特异性的基因表达来识别缓解者与未缓解者显得尤为重要[14]。
很少有文献报道胃癌SOX方案及相关差异基因表达的情况。Omura等[15]发现5种特异的miRNA与替吉奥辅助化疗后的胃癌复发情况有关。笔者[7]之前的研究已经表明,SOX新辅助化疗应用于II、III期胃癌患者具有安全性与有效性。而在新辅助化疗过程中,部分肿瘤并没有变化。因此,进展期胃癌SOX新辅助方案的药物敏感性研究是必要的。
基于功能与通路分析,缓解组与未缓解组比较,差异基因主要高表达于细胞因子相互作用及NK细胞介导的细胞毒性通路。表明SOX方案的缓解情况可能与免疫应答有关。在缓解组与未化疗组比较,基因表达也集中于NK细胞介导的细胞毒性通路上。为了排除化疗药物的药理学反应,在未缓解组与未化疗组进行比较,并没有发现差异基因表达。
有文献[16-18]报道,铂类药物及氟尿嘧啶类药物的耐药性主要与DNA损伤修复与叶酸代谢通路有关。在DNA损伤修复通路中,ERCC1的表达越高与患者预后越差[19]。本研究中,缓解组与未缓解组比较中,共发现5种差异基因。其中,HUS1编码的蛋白能够形成Rad9-Rad1-Hus1复合物,其与细胞周期停滞有关。Ishikawa等[20]报道,低表达的HUS1与胃癌高恶性程度有关。缓解组HUS1的高表达结果与该结论一致。RECQL5是RecQ解旋酶其中一种,能够维持基因组稳定性并参与错配修复过程。Futami等[21]研究表明喜树碱的化疗敏感性提高与RECQL5下调有关。本研究表明,RECQL5在生物代谢过程与核酸代谢过程起到重要作用。GADD45G是Gadd45家族中的重要成员,可作为功能性肿瘤抑制基因与治疗靶点。Ying等[22]的研究表明,GADD45G的表达与肿瘤缩小有关。本研究中GADD45G的表达可能与化疗效果的影响有关。BTG2与XRCC4的表达目前没有相关报道,仍需进一步研究。最近的研究[23-24]表明,二氢嘧啶脱氢酶(DPD)的表达与胃癌术后S-1辅助化疗有关,故本研究对叶酸代谢通路也进行分析,然而没有找到该通路的差异基因,这与笔者之前的研究结果是一致的[25]。综上所述,生物信息学技术可应用于局部进展期胃癌SOX新辅助化疗方案来评估化疗缓解情况,并且发现缓解组高表达基因与免疫信号通路相关。本研究发现的3种差异基因仍需要大型临床试验及样本进一步证实。
参考文献
[1] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China,2015[J]. CA Cancer J Clin, 2016, 66(2):115-132. doi: 10.3322/caac.21338.
[2] Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer[J]. J Clin Oncol, 2011, 29(33):4387-4393. doi: 10.1200/JCO.2011.36.5908.
[3] Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC):5-year follow-up of an open-label, randomised phase 3 trial[J].Lancet Oncol, 2014, 15(12): 1389-1396. doi: 10.1016/S1470-2045(14)70473-5.
[4] Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis[J]. Br J Surg, 2014, 101(6):653-660. doi: 10.1002/bjs.9484.
[5] Motoori M, Takemasa I, Yamasaki M, et al. Prediction of the response to chemotherapy in advanced esophageal cancer by gene expression pro filing of biopsy samples[J]. Int J Oncol, 2010,37(5):1113-1120.
[6] Washington K. 7th edition of the AJCC cancer staging manual:stomach[J]. Ann Surg Oncol, 2010, 17(12):3077-3079. doi: 10.1245/s10434-010-1362-z.
[7] Feng D, Leong M, Li T, et al. Surgical outcomes in patients with locally advanced gastric cancer treated with S-1 and oxaliplatin as neoadjuvant chemotherapy[J]. World J Surg Oncol, 2015, 13:11.doi: 10.1186/s12957-015-0444-6.
[8] Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classi fication[J]. Jpn J Surg, 1981,11(2):127-139.
[9] Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline(version 1.1)[J]. Eur J Cancer, 2009, 45(2):228-247. doi: 10.1016/j.ejca.2008.10.026.
[10] Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the uni fication of biology. The Gene Ontology Consortium[J]. Nat Genet, 2000, 25(1):25-29.
[11] Kanehisa M, Goto S, Furumichi M, et al. KEGG for representation and analysis of molecular networks involving diseases and drugs[J].Nucleic Acids Res, 2010, 38(Database issue):D355-360. doi:10.1093/nar/gkp896.
[12] Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer[J]. N Engl J Med, 2006, 355(1):11-20.
[13] Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial[J]. J Clin Oncol, 2011, 29(13):1715-1721. doi: 10.1200/JCO.2010.33.0597.
[14] Rimkus C, Friederichs J, Boulesteix AL, et al. Microarray-based prediction of tumor response to neoadjuvant radiochemotherapy of patients with locally advanced rectal cancer[J]. Clin Gastroenterol Hepatol, 2008, 6(1):53-61. doi: 10.1016/j.cgh.2007.10.022.
[15] Omura T, Shimada Y, Nagata T, et al. Relapse-associated microRNA in gastric cancer patients after S-1 adjuvant chemotherapy[J]. Oncol Rep, 2014, 31(2):613-618. doi: 10.3892/or.2013.2900.
[16] Nadin SB, Vargas-Roig LM, Drago G, et al. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in the clinical response to chemotherapy[J]. Cancer Lett, 239(1):84-97.
[17] Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents[J]. Cancer Treat Rev,2007, 33(1):9-23.
[18] Ogawa M, Watanabe M, Mitsuyama Y, et al. Thymidine phosphorylase mRNA expression may be a predictor of response to post operative adjuvant chemotherapy with S 1 in patients with stage III colorectal cancer[J]. Oncol Lett, 2014, 8(6):2463-2468.
[19] Yamada Y, Boku N, Nishina T, et al. Impact of excision repair cross-complementing gene 1 (ERCC1) on the outcomes of patients with advanced gastric cancer: correlative study in Japan Clinical Oncology Group Trial JCOG9912[J]. Ann Oncol, 2013,24(10):2560-2565. doi: 10.1093/annonc/mdt238.
[20] Ishikawa K, Ishii H, Murakumo Y, et al. Rad9 modulates me P21WAF1 pathway by direct association with p53[J]. BMC Mol Biol, 2007, 8:37.
[21] Futami K, Takagi M, Shimamoto A, et al. Increased chemotherapeutic activity of camptothecin in cancer cells by siRNA-WRN4 induced silencing of WRN helicase[J]. Biol Pharm Bull, 2007, 30(10): 1958-1961.
[22] Ying J, Srivastava G, Hsieh WS, et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors[J]. Clin Cancer Res, 2005, 11(18):6442-6449.
[23] Kim JY, Shin E, Kim JW, et al. Impact of intratumoral expression levels of fluoropyrimidine-metabolizing enzymes on treatment outcomes of adjuvant S-1 therapy in gastric cancer[J]. PLoS One,2015, 10(3):e0120324. doi: 10.1371/journal.pone.0120324.
[24] Sasako M, Terashima M, Ichikawa W, et al. Impact of the expression of thymidylate synthase and dihydropyrimidine dehydrogenase genes on survival in stage II/III gastric cancer[J]. Gastric Cancer,2015, 18(3):538-548. doi: 10.1007/s10120-014-0413-8.
[25] 李涛, 梁美霞, 袁静, 等. 氟尿嘧啶代谢因子表达水平与进展期胃癌SOX方案新辅助化疗效果[J]. 中华医学杂志, 2014, 94(2):127-130. doi:10.3760/cma.j.issn.0376-2491.2014.02.011.Li T, Liang MX, Yuan J, et al. Correlated analysis of 5 fl uorouracil metabolic enzymes with tumor response after SOX regimen neoadjuvant chemotherapy in advanced gastric cancer[J]. National Medical Journal of China, 2014, 94(2):127-130. doi:10.3760/cma.j.issn.0376-2491.2014.02.011.
