胰头癌发病隐匿,预后极差,手术根治性切除目前仍然是胰头癌患者唯一可能获得治愈和长期生存的治疗方式[1]。但因其特殊解剖位置和本身的生物学特性,早期诊断困难,手术切除率较低,很多患者甚至只有在术中才能发现肿瘤无法切除,因而有必要术前对胰头癌的可切除性进行评估[2]。尽管近年来随着影像学检查水平的提高,胰头癌术前可切除性评估的准确性已经有明显的提高,但仍具有较多的局限性。目前已经有较多研究将肿瘤标记物应用于恶性肿瘤的可切除性评估,结果显示具有较大的临床应用价值[3-5]。本研究结合104例经病理证实为胰头癌患者的临床资料,探讨术前检测肿瘤标记物水平对胰头癌可切除性评估的临床价值。
1 资料与方法
1.1 一般资料
回顾性分析2014年1月—2015年12月安徽医科大学附属省立医院收治行手术治疗的104例胰头癌患者的临床资料,其中男60例,女44例;年龄31~78岁,平均59.9岁。术前所有患者完善血常规、生化、肿瘤标记物、心肺功能及影像学等相关检查,术前增强CT等检查提示病变均位于胰头部位,无肝脏、腹膜等远处转移,无明显腹水,无肝动脉、肠系膜上动脉和腹主动脉等血管广泛受侵;全面评估全身情况要求能耐受开腹手术。本组患者均行手术探查,其中可切除组54例均行胰十二指肠切除和Child消化道重建,术后均经病理诊断为胰头癌。不可切除组50例全部经术中探查发现有肝脏、网膜等远处转移或伴血管肝动脉、肠系膜上动脉等广泛侵犯,证实不可切除;仅做姑息性胆肠内引流、胃空肠吻合或无水酒精注射腹腔神经丛毁损,对原发灶或可疑转移灶行术中活检冷冻切片检查证实为胰头癌。
1.2 检查方法
潜在相关肿瘤标记物的测定:所有患者入院后术前均常规采外周静脉血完成肿瘤标记物水平检测。采用湖州数康生物科技有限公司提供的多肿瘤标记物检测试剂盒和HD-2001A生物芯片阅读仪基于蛋白芯片-化学发光酶联免疫法检测血清中肿瘤标记物含量[(包含糖类抗原(CA19-9、CA242、CA125)和癌胚抗原(CEA)等];采用天津协和医药科技集团有限公司提供的碘125糖类抗原CA50免疫放射分析药盒和GC-1500γ放射免疫计数器(安徽中科中佳科学仪器有限公司)基于放射免疫法测定CA50含量。CA19-9正常范围0.00~35.00 kU/L,CA242正常范围0.00~20.00 kU/L,CA125正常范围0.00~35.00 kU/L,CA50正常范围0.00~25.00 U/mL,CEA正常范围0.00~5.00 ng/mL。原发肿瘤的直径大小经术后病理或影像学(增强CT或磁共振)测量。可切除性判断标准:胰腺癌肿无胰外局部及远处转移,未侵及周围重要血管及脏器,可通过手术完整彻底切除整个癌肿,在显微镜下无癌细胞残留(达到R0切除)[1]。
1.3 统计学处理
采用SPSS 19.0统计软件进行统计学分析,计量资料以均数±标准差(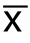 ±s)表示,计量资料比较采用t检验,计数资料比较采用Pearson χ2检验。筛选出与胰头癌可切除性相关的血清肿瘤标记物,并分别绘制受试者工作特征曲线(ROC)计算曲线下面积(AUC)并以约登指数最大确定最佳分界点,根据新的界值点计算出敏感性、特异性、阳性预测值和阴性预测值。将具有诊断能力的指标纳入Logistic回归模型,并绘制ROC曲线[6]。采用Z检验对单独检测和联合检测肿瘤标记物判断可切除性的进行比较,P<0.05为差异有统计学意义。
±s)表示,计量资料比较采用t检验,计数资料比较采用Pearson χ2检验。筛选出与胰头癌可切除性相关的血清肿瘤标记物,并分别绘制受试者工作特征曲线(ROC)计算曲线下面积(AUC)并以约登指数最大确定最佳分界点,根据新的界值点计算出敏感性、特异性、阳性预测值和阴性预测值。将具有诊断能力的指标纳入Logistic回归模型,并绘制ROC曲线[6]。采用Z检验对单独检测和联合检测肿瘤标记物判断可切除性的进行比较,P<0.05为差异有统计学意义。
2 结 果
2.1 两组临床资料比较
纳入研究的104例胰头癌患者中,可切除组和不可切除组患者在年龄、性别、入院总胆红素水平、谷丙转氨酶、谷草转氨酶等方面差异无统计学意义(均P>0.05)(表1)。两组术前血清CA50和CEA水平差异亦无统计学意义(均P>0.05);不可切除组CA19-9、CA242和CA125水平明显高于可切除组[(317.99±425.54)kU/L vs.(152.98±182.77)kU/L;(67.81±73.32)kU/L vs.(39.36±53.73)kU/L;(71.53±131.97)kU/L vs.(29.22±39.34)kU/L,P<0.05)]。在肿瘤评估方面,不可切除组原发肿瘤直径明显大于可切除组[(4.31±1.90)cm vs.(2.48±1.24)cm,P<0.05)]。
表1 104例胰头癌患者临床资料
Table 1 Clinical data of the 104 patients with pancreatic head cancer

项目 可切除组(n=54) 不可切除组(n=50) t/χ2 P年龄(岁,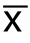 ±s) 56.17±10.68 59.44±11.63 1.49 0.14性别[n(%)]男32(59.3) 28(56.0) 1.24 0.26女22(40.7) 22(44.0)总胆红素(μmol/L,
±s) 56.17±10.68 59.44±11.63 1.49 0.14性别[n(%)]男32(59.3) 28(56.0) 1.24 0.26女22(40.7) 22(44.0)总胆红素(μmol/L,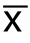 ±s) 150.57±122.49 122.83±134.41 -1.08 0.28谷丙转氨酶(IU/L,
±s) 150.57±122.49 122.83±134.41 -1.08 0.28谷丙转氨酶(IU/L,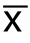 ±s) 139.02±118.84 132.76±163.95 -0.22 0.83谷草转氨酶(IU/L,
±s) 139.02±118.84 132.76±163.95 -0.22 0.83谷草转氨酶(IU/L,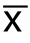 ±s) 104.60±88.73 123.11±202.96 0.59 0.55 CA19-9(kU/L,
±s) 104.60±88.73 123.11±202.96 0.59 0.55 CA19-9(kU/L,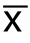 ±s) 152.98±182.77 317.99±425.54 2.58 0.01 CA50(U/mL,
±s) 152.98±182.77 317.99±425.54 2.58 0.01 CA50(U/mL,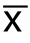 ±s) 14.25±15.73 24.10±35.14 1.78 0.08 CA242(kU/L,
±s) 14.25±15.73 24.10±35.14 1.78 0.08 CA242(kU/L,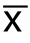 ±s) 39.36±53.73 67.81±73.32 2.05 0.04 CA125(kU/L,
±s) 39.36±53.73 67.81±73.32 2.05 0.04 CA125(kU/L,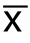 ±s) 29.22±39.34 71.53±131.97 2.10 0.04 CEA(ng/mL,
±s) 29.22±39.34 71.53±131.97 2.10 0.04 CEA(ng/mL,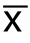 ±s) 2.83±2.36 19.89±65.87 1.12 0.27肿瘤直径(cm,
±s) 2.83±2.36 19.89±65.87 1.12 0.27肿瘤直径(cm,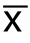 ±s) 2.48±1.24 4.31±1.90 1.26 0.02
±s) 2.48±1.24 4.31±1.90 1.26 0.02
2.2 CA19-9、CA242、CA125单独检测对胰头癌可切除性的判断
根据单项检测的ROC曲线分析(图1),CA19-9的AUC=0.667±0.059(P=0.008),当设定截断点为236.13 kU/L时Youden指数最高为0.291;CA125对胰头癌可切除性判断的最佳分界点为16.44 kU/L,此时Youden指数最大为0.291,AUC=0.678±0.058(P=0.005),两者差异均有统计学意义(P<0.05),经手工Z检验证实CA19-9与CA125对胰头癌可切除性判断具有相似的诊断效率。而CA242的AUC=0.609±0.062,经Z检验结果显示,单项检测CA242对胰头癌可切除性诊断无判别能力(Z=1.76,P=0.085)(表2),未将其纳入后续基于Logistic回归模型的联合诊断分析。
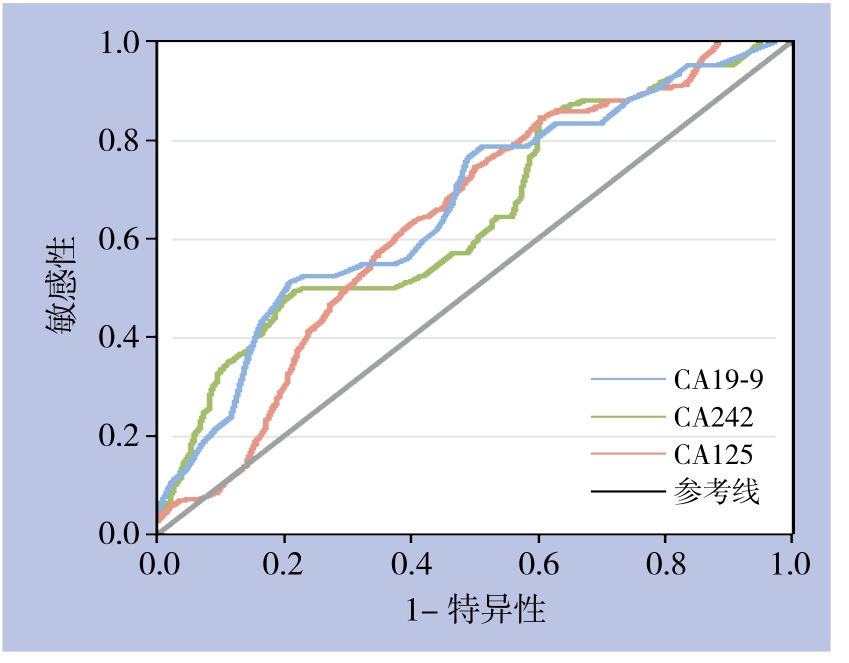
图1 CA19-9、CA242和CA125单独检测的ROC曲线
Figure 1 ROC curves of independent detection of CA19-9,CA242 and CA125
表2 ROC曲线分析相关指标
Table 2 ROC curve analysis and related variables
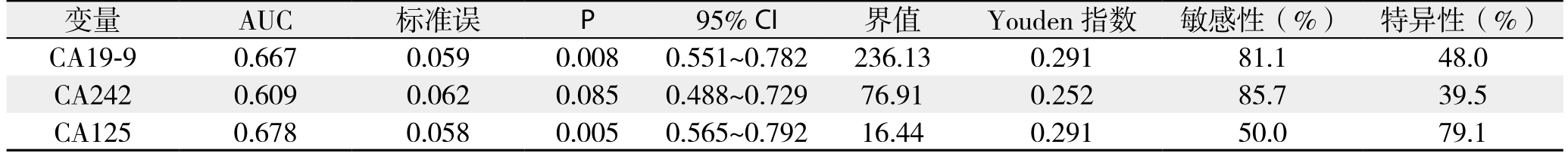
变量 AUC 标准误 P 95% CI 界值 Youden指数 敏感性(%) 特异性(%)CA19-9 0.667 0.059 0.008 0.551~0.782 236.13 0.291 81.1 48.0 CA242 0.609 0.062 0.085 0.488~0.729 76.91 0.252 85.7 39.5 CA125 0.678 0.058 0.005 0.565~0.792 16.44 0.291 50.0 79.1
2.3 基于Logistic回归模型的联合诊断对胰头癌可切除性的判断
以可切除性为因变量,CA19-9、CA125为自变量,选用 logit 连接函数建立Logistic回归模型:logit(P)=-0.563+0.00092×YCA19-9+0.0069×YCA125。对该回归方程进行变换可得到新的预测指标:X(y)=YCA19-9+7.5YCA125,并将各观察指标代入该方程求得新预测指标。根据绘制ROC曲线(图2),预测指标的AUC=0.684±0.058,差异具有统计学意义(P=0.006),具有的诊断价值。然而Z检验提示,相比于单独诊断,AUC值差异无统计学意义(P>0.05)。
2.4 CA19-9、CA125平行和系列联合试验对胰头癌可切除性的判断
分别以236.13 kU/L、16.44 kU/L为 CA19-9和 CA125对胰头癌可切除性判断的最佳临界点,单独CA19-9检测、单独CA125检测、平行试验联合检测(有一项超过新界值,即CA19-9>236.13 kU/L或CA125>16.44 kU/L作为阳性,两项均不超过新界值为阴性)、系列试验联合检测(两项均超过新界值,即两项均超过新界值作为阳性,CA19-9<236.13 kU/L或CA125<16.44 kU/L为阴性)对可切除性预测的敏感性、特异性、漏诊率、误诊率、阳性预测值、阴性预测值阳性似然比、阴性似然比见表3。若采用平行试验联合检测法,可以提高检测的敏感性(94.4%)和阴性预测值(84.2%),但特异性(32.0%)和阳性预测值(60.0%)下降;若采用系列试验联合检测法,则降低了敏感性(37.0%)和阴性预测值58.0%),提高了特异性(94.0%)和阳性预测值(86.9%)。
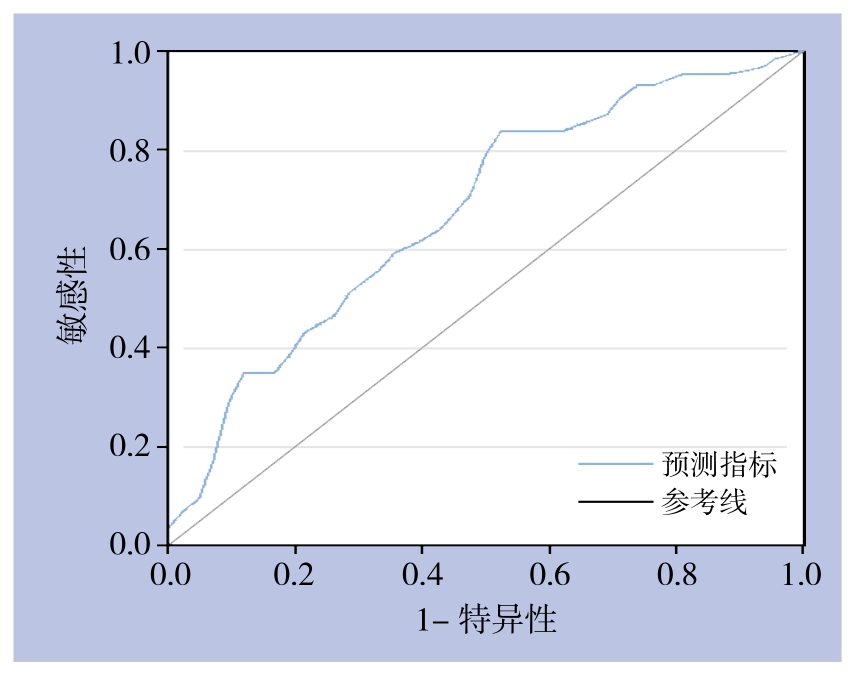
图2 基于Logistic模型联合检测的ROC曲线
Figure 2 ROC curve of combined detection based on Logistic regression model
表3 CA19-9、CA125及其联合检测预测胰头癌可切除性的灵敏度及特异性
Table 3 Sensitivity and speci ficity of CA19-9 and CA125 and their combination for predicting resectability of pancreatic head cancer
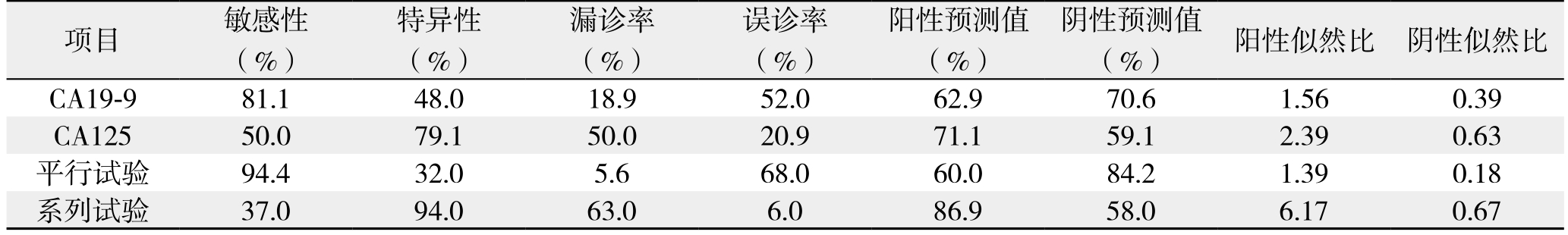
项目 敏感性(%)特异性(%)漏诊率(%)误诊率(%)阳性预测值(%)阴性预测值(%) 阳性似然比 阴性似然比CA19-9 81.1 48.0 18.9 52.0 62.9 70.6 1.56 0.39 CA125 50.0 79.1 50.0 20.9 71.1 59.1 2.39 0.63平行试验 94.4 32.0 5.6 68.0 60.0 84.2 1.39 0.18系列试验 37.0 94.0 63.0 6.0 86.9 58.0 6.17 0.67
3 讨 论
根治性胰十二指肠切除术是目前治疗胰头癌及其他壶腹部肿瘤的最有效的手术方式[7],而胰头癌的预后往往较差,可接受手术切除者仅占15%~20%[8],而有机会根治性切除的病例尽量不要错失手术的机会,因此术前可切除性评估尤为重要,对每个患者都应进行详细准确的术前评估,可减少不必要的“剖腹探查”。目前在胰头癌的可切除性判断中,仍首选无创影像学检查,但即使是对可切除性判断最有效的增强 CT 准确率也只有60%~70%左右[9-10]。目前已有研究[11-14]发现肿瘤标记物不仅对恶性肿瘤诊断有较好的参考价值,而且与肿瘤的进展、侵袭转移密切相关,在评价肿瘤可切除性方面具有重要意义。本研究选取了5种与胰头癌相关的肿瘤标记物,探讨其在胰头癌可切除性评估的临床价值。
本研究发现,术前血清CA50和CEA水平与胰头癌可切除性无明显关系。CA19-9、CA242和CA125水平与胰头癌可切除性均具有显著性关系。进一步采用ROC曲线分析显示术前血清CA242水平检测对胰头癌可切除性诊断不具有判别能力,而血清CA19-9和CA125对胰头癌可切除性判断的最佳临界点分别为236.13 kU/L、16.44 kU/L。在此界值下,单独检测血清 CA19-9和 CA125曲线下面积均在0.65左右,这表明CA19-9和CA125对胰头癌的可切除性判断有一定准确性。单独检测CA19-9对判断胰头癌可切除性敏感性优于单独检测CA125,但误诊率高达52%,而单独检测CA125误诊率低,但敏感性不高,因此两者均不是诊断胰头癌可切除性的理想指标。为此将CA19-9、CA125纳入Logistic回归模型,按回归系数加权建立新的预测指标,然而基于Logistic回归模型的新预测指标并不能明显提高诊断效率,3项诊断试验的诊断效率差异无统计学意义。CA19-9是一种高分子糖蛋白,目前认为血清 CA19-9水平升高与胰腺癌肿瘤负荷密切相关[15],在胰腺癌的诊断和预后方面价值较高,但其表达依赖于Lewis血型抗原的表达,易受黄疸、炎症等因素的影响[16],因此这可能导致诊断过程的假阳性和假阴性,从而降低了CA19-9的诊断效率。CA125是一种广谱的肿瘤标记物,是上皮性卵巢癌最敏感的标记物[17],近年来有研究[18-19]表明血清CA125升高可能与胰腺癌肿瘤转移负荷密切相关,在胰腺癌的可切除性判断中可能具有较高价值。本研究表明血清CA125水平对胰头癌可切除性判断的最佳临界点为16.44 kU/L,尽管目前血清CA125诊断卵巢癌最常用的截断值为35.0 kU/L,但研究[20-22]表明低于水平的CA125对胰腺癌的诊断和预后具有重要价值,这与本研究结果是相符的。另外本研究结果显示:平行试验联合检测法提高了检测的敏感性、阴性预测值,可明显减少漏诊率,而特异性和阳性预测值下降。系列试验联合检测法提高了检测的特异性、阳性预测值,而敏感性和阴性预测值下降。因此联合检测可以很好的综合CA19-9和CA125单独检测的敏感性和特异性,在整体效果上联合检测法优于单项检测[23]。
然而,尽管检测CA19-9和CA125水平能一定程度上术前预测胰头癌可切除性,提供了除影像学以外的另一种较廉价、快捷的诊断措施,但单独诊断效率明显偏低,因此术前判断胰头癌能否完整切除,需结合患者临床表现、术前影像学资料如增强CT等进行综合分析后进一步判定,目前认为肿瘤标记物仅作为辅助指标判断胰头癌的可切除性,特别是在对于临界可切除胰腺癌的评估可能具有重要参考价值[24-25]。另外,本研究为回顾性分析,且纳入样本量较少等混杂因素可能影响研究结果,因此肿瘤标记物对胰头癌的可切除性的评估价值需要大样本、多中心的前瞻性研究进一步验证。
参考文献
[1]杨尹默.胰腺癌外科治疗的现状、存在问题与展望[J].中国普通外科杂志,2016,25(9):1231–1235.doi:10.3978/j.issn.1005–6947.2016.09.001.Yang YM.Surgical managements of pancreatic cancer:current status and future directions[J].Chinese Journal of General Surgery,2016,25(9):1231–1235.doi:10.3978/j.issn.1005–6947.2016.09.001.
[2]张太平,展翰翔,赵玉沛.做好"胰腺癌术前可切除性评估"[J].中华肝胆外科杂志,2010,16(2):81–84.doi:10.3760/cma.j.issn.1007–8118.2010.02.001.Zhang TP,Zhan HX,Zhao YP.Full preparation for preoperative evaluation of resectability of pancreatic cancer[J].Chinese Journal of Hepatobiliary Surgery,2010,16(2):81–84.doi:10.3760/cma.j.issn.1007–8118.2010.02.001.
[3]罗国培,龙江,刘辰,等.血清癌抗原19-9水平对胰腺癌可切除性判断的临床意义[J].中华肝胆外科杂志,2012,18(6):436–438.doi:10.3760/cma.j.issn.1007–8118.2012.06.012.Luo GP,Long J,Liu C,et al.The role of preoperative CA19–9 level in predicting resectability of pancreatic cancer[J].Chinese Journal of Hepatobiliary Surgery,2012,18(6):436–438.doi:10.3760/cma.j.issn.1007–8118.2012.06.012.
[4]Fujioka S,Misawa T,Okamoto T,et al.Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19–9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma[J].J Hepatobiliary Pancreat Surg,2007,14(6):539–544.
[5]Kim YC,Kim HJ,Park JH,et al.Can preoperative CA19–9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma?[J].J Gastroenterol Hepatol,2009,24(12):1869–1875.
[6]刘润幸.使用SPSS作多变量观察值的ROC曲线分析[J].中国公共卫生,2003,19(9):1151–1152.doi:10.3321/j.issn:1001–0580.2003.09.078.Liu RX.ROC analysis of multivariate observation values by using SPSS[J].Chinese Journal of Public Health,2003,19(9):1151–1152.doi:10.3321/j.issn:1001–0580.2003.09.078.
[7]刘双海,周一夫,陈胜,等.胰十二指肠切除手术流程及方法的改进[J].中国普通外科杂志,2011,20(3):234–237.Liu SH,Zhou YF,Chen S,et al.Improvement of operation technological process and method in pancraticoduodenectomy[J].Chinese Journal of General Surgery,2011,20(3):234–237.
[8]Saif MW.Advancements in the management of pancreatic cancer:2013[J].JOP,2013,14(2):112–118.doi:10.6092/1590–8577/1481.
[9]石宁,戴梦华.如何提高胰腺癌的手术切除率[J].中华临床医师杂志:电子版,2014,8(24):4320–4324.doi:10.3877/cma.j.issn.1674–0785.2014.24.001.Shi N,Dai MH.How to improve the R0 resection of pancreatic cancer?[J].Chinese Journal of Clinicians:Electronic Edition,2014,8(24):4320–4324.doi:10.3877/cma.j.issn.1674–0785.2014.24.001.
[10]Morgan DE,Waggoner CN,Canon CL,et al.Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy:a challenge for MDCT[J].AJR Am J Roentgenol,2010,194(3):615–622.doi:10.2214/AJR.08.1022.
[11]潘树波,赵红川,谢坤,等.增强CT联合PET/CT在胰腺癌可切除性评价中的价值[J].中华肝胆外科杂志,2013,19(10):726–729.doi:10.3760/cma.j.issn.1007–8118.2013.10.002.Pan SB,Zhao HC,Xie K,et al.Enhanced CT and 18 F-FDG PET/CT in evaluating resectability of pancreatic cancer[J].Chinese Journal of Hepatobiliary Surgery,2013,19(10):726–729.doi:10.3760/cma.j.issn.1007–8118.2013.10.002.
[12]Liao Q,Zhao YP,Yang YC,et al.Combined detection of serum tumor markers for differential diagnosis of solid lesions located at the pancreatic head[J].Hepatobiliary Pancreat Dis Int,2007,6(6):641–645.
[13]Kim JR,Jang JY,Kang MJ,et al.Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19–9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas[J].J Hepatobiliary Pancreat Sci,2015,22(9):699–707.doi:10.1002/jhbp.275.
[14]Cao J,Fu Z,Gao L,et al.Evaluation of serum D-dimer,fibrinogen,and CA19–9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma[J].World J Surg Oncol,2017,15(1):48.doi:10.1186/s12957–017–1104–9.
[15]Luo G,Xiao Z,Long J,et al.CA125 is superior to CA19–9 in predicting the resectability of pancreatic cancer[J].J Gastrointest Surg,2013,17(12):2092–2098.doi:10.1007/s11605–013–2389–9.
[16]O'Brien DP,Sandanayake NS,Jenkinson C,et al.Serum CA19–9 is signi ficantly upregulated up to 2 years before diagnosis with pancreatic cancer:implications for early disease detection[J].Clin Cancer Res,2015,21(3):622–631.doi:10.1158/1078–0432.CCR–14–0365.
[17]范艳红,柏淑霞,王伟红.血清肿瘤标志物CA199、CA125、CA242、CEA检测对不同分期胰腺癌相关性的分析[J].中国医药指南,2014,12(12):238–239.Fan YH,Bai SX,Wang WH.Correlation analysis of detection of serum tumor markers CA199,CA125,CA242 and CEA in different stages of pancreatic cancer[J].Guide of China Medicine,2014,12(12):238–239.
[18]蒋萱,王东,杨雪琴,等.多肿瘤标志物蛋白芯片检测中CA125对恶性肿瘤的诊断价值[J].中国肿瘤临床,2012,39(9):607–610.doi:10.3969/j.issn.1000–8179.2012.09.029.Jiang X,Wang D,Yang XQ,et al.Diagnostic Values of Carbohydrate Antigen 125 in Multiple Tumor Marker Protein Chip Detection Systems for Malignant Tumors[J].Chinese Journal of Clinical Oncology,2012,39(9):607–610.doi:10.3969/j.issn.1000–8179.2012.09.029.
[19]Xu HX,Liu L,Xiang JF,et al.Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19–9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma[J].Surgery,2017,161(2):373–384.doi:10.1016/j.surg.2016.08.005.
[20]Liu L,Xiang J,Chen R,et al.The clinical utility of CA125/MUC16 in pancreatic cancer:A consensus of diagnostic,prognostic and predictive updates by the Chinese Study Group for Pancreatic Cancer (CSPAC)[J].Int J Oncol,2016,48(3):900–907.doi:10.3892/ijo.2015.3316.
[21]Menczer J,Ben-Shem E,Golan A ,et al.The Signi ficance of Normal Pretreatment Levels of CA125 (<35 U/mL) in Epithelial Ovarian Carcinoma[J].Rambam Maimonides Med J,2015,6:e0005.doi:10.5041/RMMJ.10180.
[22]Liu L,Xu HX,Wang WQ,et al.Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden[J].Oncotarget,2016,7(5):5943–5956.doi:10.18632/oncotarget.6819.
[23]Duraker N,Hot S,Polat Y,et al.CEA,CA19–9,CA125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice[J].J Surg Oncol,95:142–147.doi:10.1002/jso.20604.
[24]Ramacciato G,Nigri G,Petrucciani N,et al.Pancreatectomy with Mesenteric and Portal Vein Resection for Borderline Resectable Pancreatic Cancer:Multicenter Study of 406 Patients[J].Ann Surg Oncol,2016,23(6):2028–2037.doi:10.1245/s10434–016–5123–5.
[25]曲凤智,王刚,孙备,等.临界可切除胰腺癌新辅助治疗的研究进展[J].中华肝胆外科杂志,2015,21(3):206–209.doi:10.3760/cma.j.issn.1007–8118.2015.03.016.Qu FZ,Wang G,Sun B,et al.Updates on neoadjuvant therapy in borderline resectable pancreatic cancer[J].Chinese Journal of Hepatobiliary Surgery,2015,21(3):206–209.doi:10.3760/cma.j.issn.1007–8118.2015.03.016.